At ACM Global Laboratories, we understand the critical importance of time when it comes to clinical trials - especially as 2024 comes to an end.
That's why we're excited to offer you an accelerated path to success with our streamlined process.
Need kits on site quickly? We can have them ready in as little as 2 weeks*. Our efficient expedite process is designed to uphold the highest standards of quality and compliance, ensuring that your timelines are met without compromise.
If you're ready to fast-track your study and explore how these enhancements can benefit your specific needs, click below to get started. Let's accelerate together!
At ACM Global Laboratories, we specialize in both expedited and rescue study processes, ensuring your clinical trials stay on track. Whether you need to fast-track timelines or navigate unforeseen challenges, our dedicated teams provide tailored support and solutions. Explore our approaches to ensure your study's success!

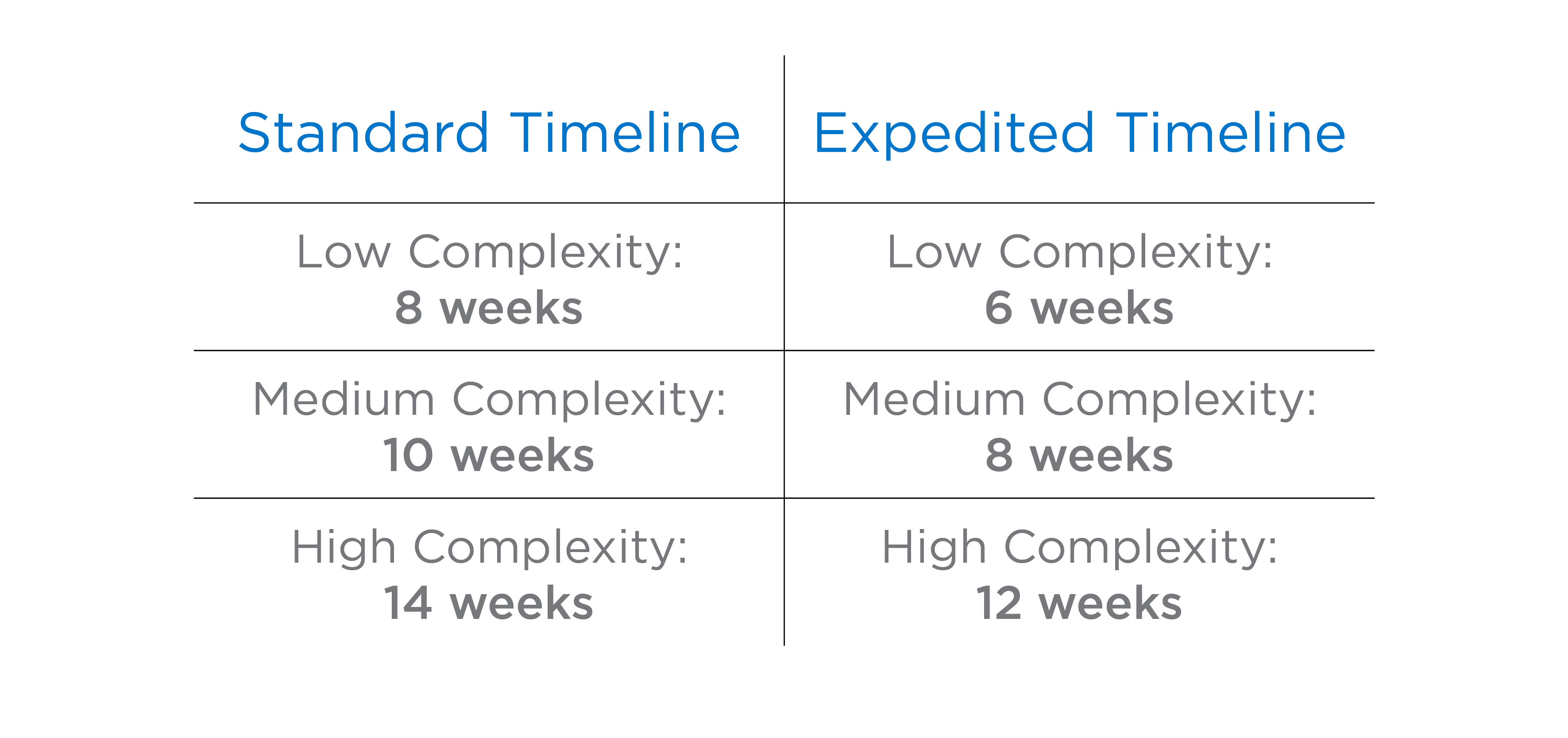
Our expedited process can save you at least two weeks, ensuring faster results and submissions.

We tailor each expedited study to your specific needs, aligning resources for optimal efficiency.

ACM's Rescue Team provides personalized plans, addressing issues and ensuring study continuity and quality.

We conduct root cause analysis to implement effective solutions and align resources for successful outcomes.
To learn more, download our Expedite Study Capabilities and Rescue Study Considerations sell sheets.
These resources provide valuable insights into how we streamline study timelines and address challenges in ongoing trials. Get the details you need to make informed decisions and ensure the success of your clinical research.
*Study complexity permitting. Studies must be signed within Q4 and are subject to laboratory operational capacity.