At ACM Global Laboratories, we are here to help facilitate your clinical trial study in an expedited fashion.
We assess each expedited clinical study request individually to ensure we are aligning the right resources to get your study moving quickly to deliver according to submission timelines, working with you to create a customized plan focused on the quality and expertise your study demands.
The ACM expedited clinical trials process shaves at least TWO WEEKS off of our standard timelines.




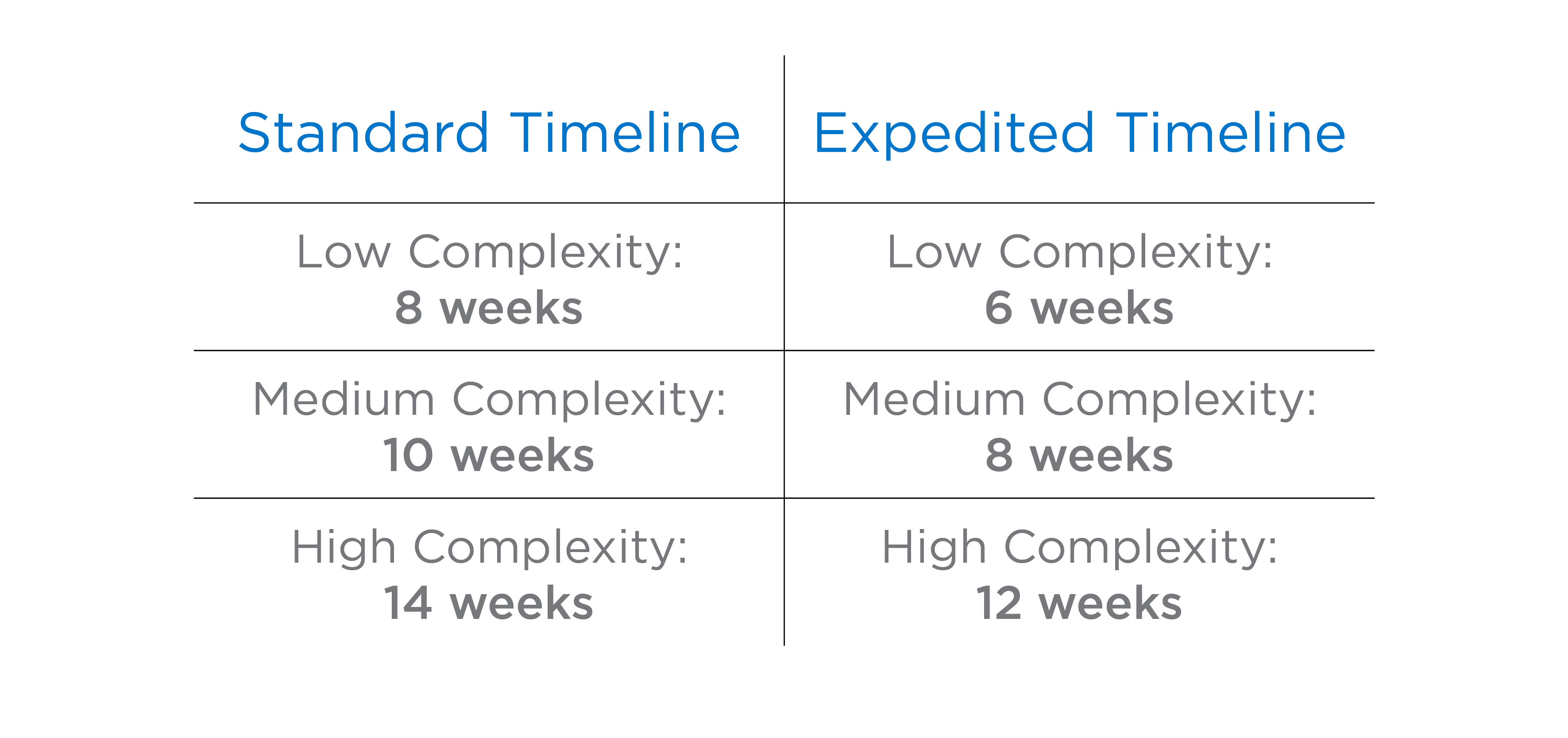
Because each study is unique to the needs of the client, timeframes and exact specifics might differ on a case-by-case basis.
We understand each study is unique, and each expedited process must be carefully crafted to fulfill distinctive needs. Together, we work with your team to fast-track your clinical trial study to completion and results.