Article
The medical and scientific communities continue working around the clock in response to the novel coronavirus (COVID-19) pandemic. Medical diagnostic testing to identify patients that have contracted the infection is essential for successfully stemming the tide of this global public health threat.
On January 11, 2020, when investigators in China released the genetic sequence of SARS-CoV-2, the virus that causes COVID-19 infection, the US Centers for Disease Control and Prevention (CDC) and laboratories around the world began developing the first COVID-19 laboratory test in the US to detect the virus in patient samples. This test was released to state laboratories on February 4, 2020.
Under Emergency Use Authorization (EUA), the US Food and Drug Administration (FDA) approved two main categories of COVID-19 tests that are produced or administered by public health, commercial, and hospital system laboratories around the country. Tests may only be performed by facilities with Clinical Laboratory Improvement Amendments (CLIA) accreditation.
The first type is a molecular test that identifies genetic material derived from the SARS-CoV-2 virus itself.
The second category of COVID-19 testing available, not covered in this article, detects antibodies, which are proteins present in the blood, serum, or tissues. Antibody blood tests, also called serologic tests, indicate an immune response produced by individuals that were exposed to the SARS-CoV-2 virus.
These two testing categories are performed using vastly different methods and each has unique benefits, limitations, and applications. This article provides an overview of the technology platform used to produce the majority of molecular diagnostic tests that detect the SARS-CoV-2 virus and explores variations of the different molecular testing approaches currently available.
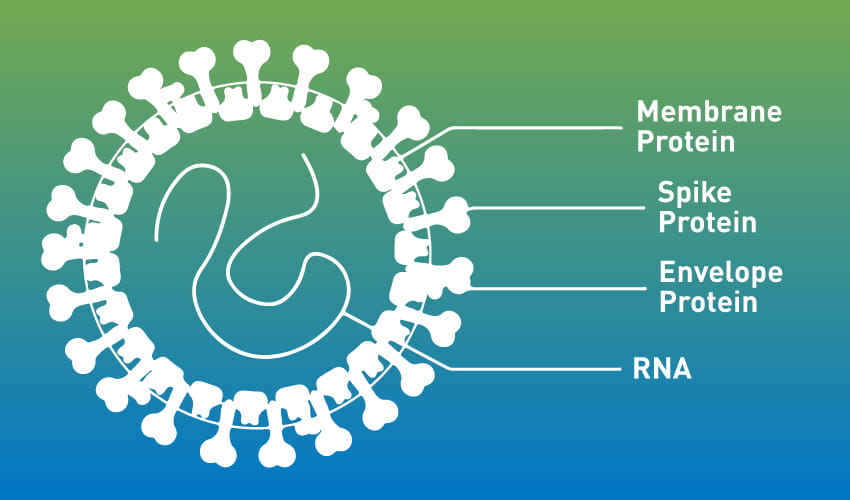
Figure 1. Structure of SARS-CoV-2
Due to its high degree of selectivity, the gold standard test for COVID-19 is performed using a method called real-time Reverse Transcriptase Polymerase Chain Reaction (rRT-PCR) that detects SARS-COV-2-specific ribonucleic acid (RNA) in a positive patient sample. The majority of COVID-19 tests developed employ rRT-PCR.
This methodology is the technique of choice for detecting, quantifying, and typing viral pathogens because it provides fast and high-throughput detection of target complementary DNA sequences in different matrices. Isothermal nucleic acid amplification is a less-frequently used molecular test for COVID-19 that is available.
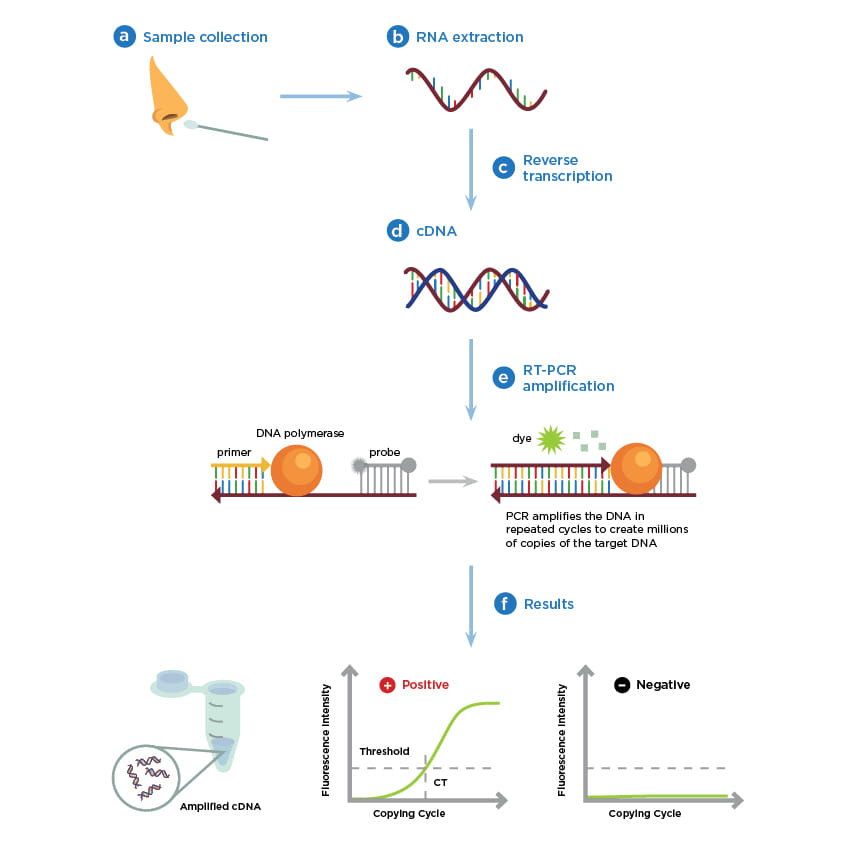
The following illustration outlines the basic steps for viral RNA testing by rRT-PCR that enables the detection of infections caused by coronaviruses, influenza, human immunodeficiency virus, and numerous other microbial pathogens.
Proper specimen collection technique is one of the most important steps in the diagnosis of an infectious disease such as COVID-19. As outlined by the illustration, (a) the rRT-PCR testing process begins with collecting an upper respiratory specimen, preferably using a nasopharyngeal swab taken from a suspected individual. For information about alternative specimen types for COVID-19 testing view these CDC Guidelines.
Using one of several EUA-approved COVID-19 testing kits that are each designed to run on a compatible instrument, (b) RNA is extracted from the sample and subsequently converted from single-stranded viral RNA to (c) a complementary DNA (cDNA) intermediate using an enzymatic process called reverse transcriptase. Once converted into (d) double-stranded DNA, the addition of (e) a set of short, target-specific DNA primers, a mixture of nucleotide subunits, and an enzyme called Taq Polymerase are required to copy the target DNA. A reporter probe labeled with a fluorescent dye is used to monitor real-time target DNA amplification.
To correctly interpret the results, the assay, or investigative lab procedure, must include both internal positive and negative control samples run in parallel with the test samples. An increase in the fluorescence signal, or light intensity, corresponds to an increase in the number of viral cDNA copies that are amplified. The test is positive if the fluorescent signal exceeds the threshold of the assays expected background signal (Figure 1e). Conversely, a negative sample would remain below the background fluorescence threshold of the assay.
Figure 2. Simplified real-time RT PCR workflow
The FDA has authorized dozens of companies to produce and distribute molecular tests to public health, commercial, and clinical laboratories that have the CLIA accreditation to perform EUA diagnostic testing for COVID-19. Frequently, nasopharyngeal swabs are obtained at healthcare facilities or drive-through testing sites, then transported to a laboratory for analysis.
Healthcare systems with on-site testing capabilities can provide relatively fast turn-around times compared to facilities that must send samples to third party labs for testing. While most rRT-PCR tests take 4 to 6 hours to process on average, depending on where the test is administered, the particular testing platform that is used, and the volume of samples, it may take from 1 to 7 days before the clinical review can be completed and the results are reported.
Currently, the demand for COVID-19 molecular testing remains high; so, while test manufactures have increased their production capacity, it remains unknown if they can stay in step with the global demand. Presently, healthcare workers, first responders, people at risk for severe illness, and patients with worsening symptoms receive molecular testing for COVID-19. In an effort to reduce the burden on clinical laboratories and respond to supply chain shortages of critical testing supplies and reagents, limited options for point-of-care and at-home COVID-19 testing and alternative sample collection methods have been FDA approved.
In March, the FDA issued EUA status for Abbott’s ID NOW™ platform, the first available molecular point-of-care COVID-19 test. Unlike rRT-PCR, which requires multiple heating and cooling—or thermal cycle—steps to amplify DNA, the novel test makes use of isothermal nucleic acid amplification technology, which allows the assay to run at a constant temperature—making it relatively faster to perform. The portable instrument is designed for use in physician’s offices, urgent care facilities, and drive-through testing sites rather than in a clinical laboratory and is reported to provide positive results within 5 minutes and negative results in 13 minutes.
To address the issue with a global shortage of swabs for patient sampling, the FDA granted EUA status to Rutgers’ RUCDR Infinite Biologics (RUCDR) in Piscataway, New Jersey, and their collaborators. Their COVID-19 diagnostic test uses patient-collected saliva, rather than provider-collected specimens obtained with nasopharyngeal swabs. This PCR-based test is currently available only in certain counties in New Jersey but RUCDR is partnering with industry to expand the use of their novel test.
On April 20, 2020, LabCorp received EUA granted by the FDA for the first at-home molecular COVID-19 test available to healthcare workers and first responders. The Pixel test kit utilizes a nasal swab that the user returns to the lab for testing. In a recent update, the FDA indicated that it is working with at-home test providers in an attempt to increase safe and accurate options for COVID-19 testing, which may include at-home collection methods.
While molecular testing is a powerful and indispensable tool in the arsenal for identifying infectious diseases, it is necessary to consider limitations around the use of these tests. For the analytical sensitivity of rRT-PCR, the limit of detection (LoD) of the assay dictates the lowest concentration of viral RNA that can be detected. For COVID-19 assays, the LoD has been reported at levels lower than 10 genome copies per reaction (cp/mL), meaning that some tests are highly sensitive and can detect low levels of viral RNA in a sample. However, the LoD of COVID-19 tests varies widely depending on the sample collection method used, the kit that is employed, or the type of instrument that is used. These kinds of variations may cause test result inconsistencies.
False negative test results may arise due to low analytical sensitivity or other issues that may include challenges with sample collection or preparation. Molecular COVID-19 tests may also produce false positive results due to assay quality control issues. It is important to remember that both positive and negative COVID-19 test results must be interpreted in conjunction with information available from clinical evaluation of the patient and patient history. Importantly, the rRT-PCR test only indicates if the novel coronavirus was present at the time the sample was collected. The test is unable to rule out if an individual previously had the infection and recovered or if the patient under investigation is at an early stage of infection and will go on to develop symptoms or become an asymptomatic carrier.
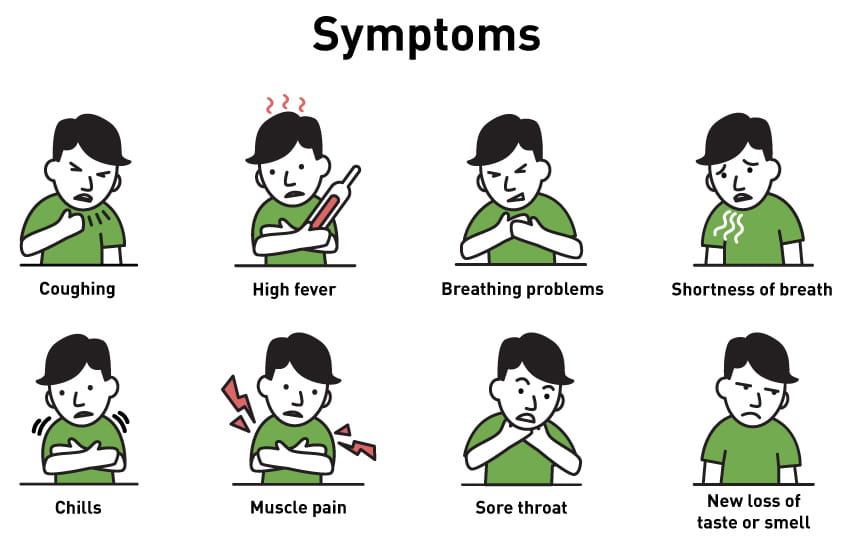
The World Health Organization and the CDC report that the most common symptoms of COVID-19 are fever, tiredness, and dry cough. Additionally, some patients may experiences these symptoms: a temperate of 100 or above, shortness of breath, chills, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell. Symptoms tend to be mild and occur gradually in some patients; however, symptoms may escalate in severity and some patients may present with high fever, shortness of breath, and severe cough.
Individuals with concerns about the status of their health or the health of a family member should contact their healthcare provider or local public health office for advice about the current CDC guidelines for COVID-19 testing, when to self-quarantine at home, and when to access urgent medical attention.
To stay abreast of the latest COVID-19 epidemiological information these websites update relevant data daily:
Global and national efforts to decrease the spread of COVID-19 have been aided by the availability of established diagnostic technologies such as rRT-PCR. In 1985, the polymerase chain reaction was first invented by Dr. Kary Mullis who was awarded the Nobel Prize in 1993.
The evolution and optimization of the original technology over the decades has provided researchers with a valuable tool for responding to public health crises. During the 2003 Severe Acute Respiratory Syndrome (SARS) outbreak, it took approximately 6 months to identify and sequence the SARS-CoV virus and develop PCR primers and probes for rRT-PCR testing. In the wake of COVID-19, scientists used the same techniques to identify SARS-CoV-2 and rapidly develop a molecular test. Officials in China first reported cases of the novel coronavirus in December 2019; the vial genome was sequenced in January 2020, and the first COVID-19 molecular tests were developed the same month.
The ability to rapidly develop and mobilize molecular testing is the world’s first line of defense in curbing infectious disease outbreaks. The availability of diagnostic testing has enabled healthcare workers to direct resources and efforts to patients with COVID-19, which can help slow the spread of the disease and lower mortality.